Search Results
Results for: 'activation energy'
Activation Energy - Valence Electrons
By: HWC, Views: 6091
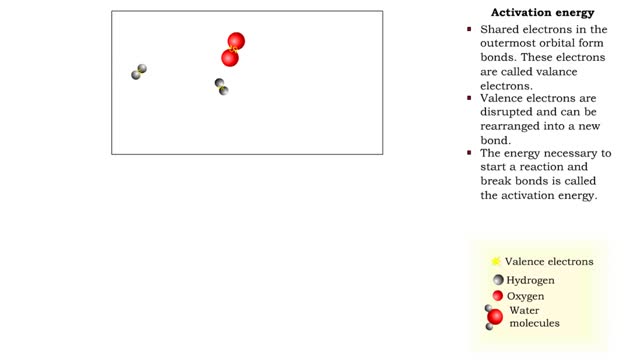
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have...
Helper T cell receptors, activation, proliferation, differentiation & action
By: HWC, Views: 6745
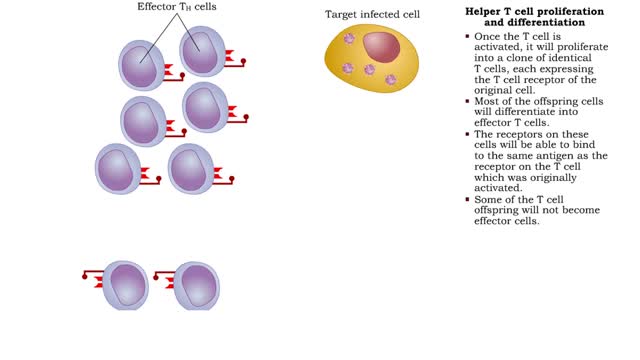
• Most cells which have CD4 on their surface become Helper T cells (TN cells). • The CD4 1 cells only recognize a foreign antigen when it is presented with an antigen presenting immune cell (APC) that includes MHC-II protein. • The Helper T cell antigen receptor must match the presented...
Cytotoxic T cell receptors, activation, proliferation, differentiation & action
By: HWC, Views: 6985
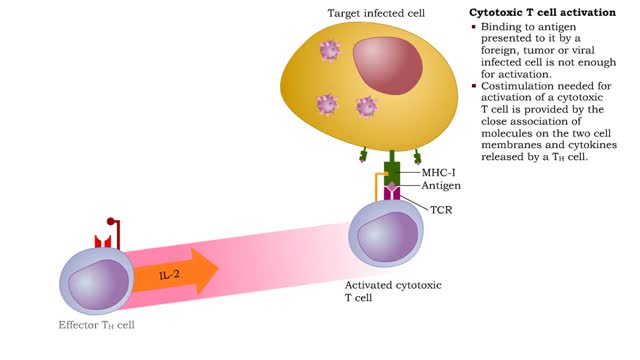
• Most cells which have CD8 on their surface become cytotoxic T cells (Tc cells). • CD8 T cells recognize a foreign antigen when it is presented in conjunction with the protein, MHC-I. • Nearly all nucleated cells in the body express MHC-I molecules. • T cells that recognize self-pe...
By: HWC, Views: 7462
What Are Antibodies? Antibodies, also known as immunoglobulins, are Y-shaped proteins that are produced by the immune system to help stop intruders from harming the body. When an intruder enters the body, the immune system springs into action. These invaders, which are called antigens, can be vi...
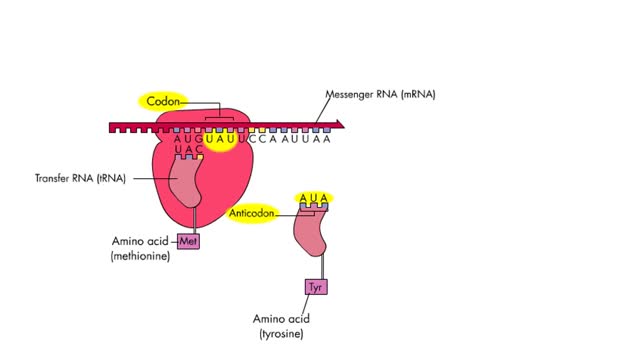
The 4 steps of translation Animation
By: HWC, Views: 2490
Translation is the process of formation of a polypeptide chain according to codon present in mRNA. The four steps of translation are: Activation or charging of tRNA Initiation – recognition of start codon, binding of ribosomal subunits to mRNA and formation of initiation complex with Met-tR...
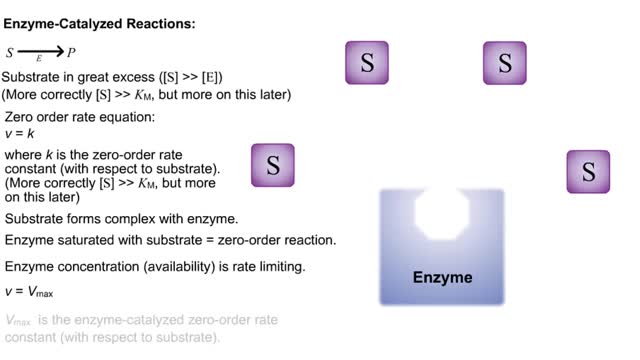
By: HWC, Views: 6129
S P Substrate in great excess ([S] -- [E]) (More correctly [S] -- KM, but more on this later) Zero order rate equation: v = k where k is the zero-order rate constant (with respect to substrate). (More correctly [S] -- KM, but more on this later) Substrate forms complex with enzyme. ...
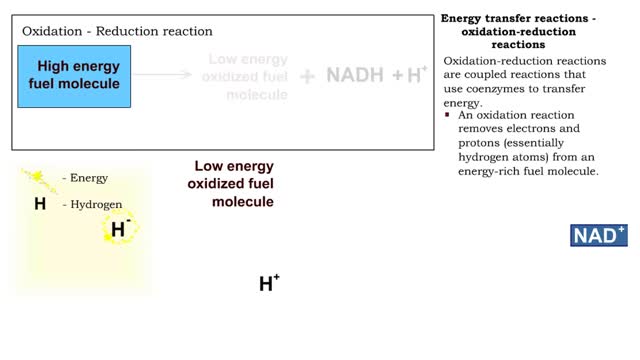
Types of energy transfer reactions: oxidation-reduction reactions and ATP generation reactions
By: HWC, Views: 7187
■ Metabolism balances anabolic and catabolic reactions. ■ Anabolism is energy transfer from ATP to simpler molecules in order to build them up into larger, more complex molecules. ■ Catabolism is breaking down larger, more complex molecules, usually to transfer energy from them in order...
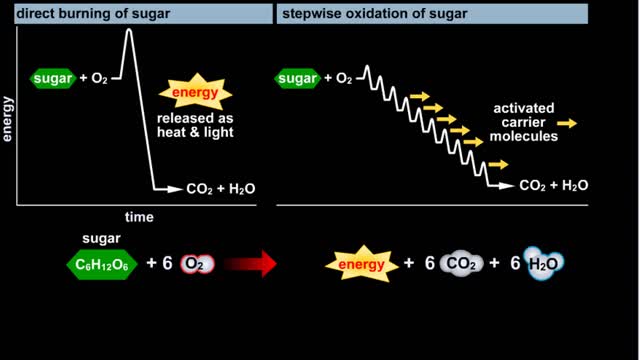
Glycolysis - Introduction to ATP and the burning of sugar
By: HWC, Views: 6735
Do you use sugar with your coffee or tea? Or do you occasionally drink a sport or soft drink? As millions of people do each day, they obtain energy from the sugar added or contained in these drinks. How can we understand this concept of energy within a sugar molecule? Let's take a tablespoon ...
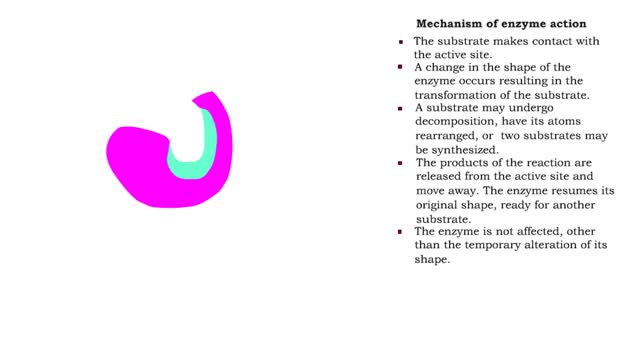
By: HWC, Views: 6538
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
Advertisement